When you pick up a prescription at the pharmacy and see a brand name like Lyrica replaced by pregabalin, you’re seeing the result of something called an ANDA. It’s not a drug itself-it’s the paperwork that lets a generic version hit the shelves. And it’s one of the most powerful tools in American healthcare for keeping medicine affordable.
What exactly is an ANDA?
An Abbreviated New Drug Application (a regulatory submission to the U.S. Food and Drug Administration (FDA) to approve a generic drug by proving it’s identical in performance to a brand-name drug) is the official form a company fills out to get FDA approval to sell a generic version of a brand-name medication. The word "abbreviated" doesn’t mean it’s easy-it means they don’t have to start from scratch.
Before ANDAs existed, every new drug-no matter how similar-needed full clinical trials. That meant years of testing and millions in costs. The Hatch-Waxman Act (a 1984 U.S. law that created the ANDA pathway to balance generic access with patent protections) changed that. Signed into law by President Ronald Reagan, it let generic manufacturers skip repeating expensive human trials. Instead, they just had to prove their version worked the same way as the original.
How does an ANDA work?
To get approval, a generic company must show four things:
- Same active ingredient-The generic must contain the exact same medicine as the brand. No more, no less. If the brand is 10 mg of lisinopril, the generic must be 10 mg of lisinopril.
- Same dosage form-Tablet, capsule, injection, patch? It has to match. A pill can’t become a liquid unless it’s a different product entirely.
- Same strength and route-If the brand is taken orally once daily, the generic must be too. Same dose, same schedule.
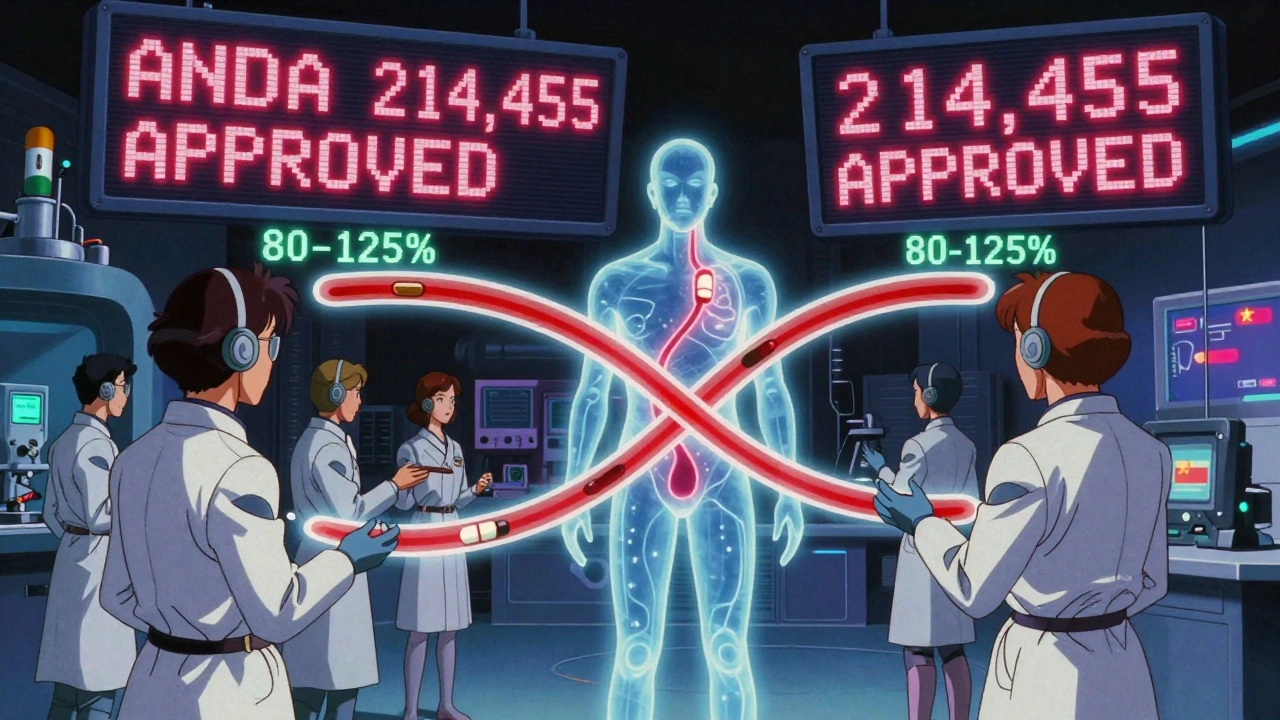
- Bioequivalence-This is the real test. The generic must absorb into the bloodstream at the same rate and to the same extent as the brand. In practice, that means testing 24-36 healthy volunteers in a controlled study. The results must show that the amount of drug in the blood (measured by AUC and Cmax) falls within 80-125% of the brand’s levels. That’s the FDA’s accepted range for therapeutic equivalence.
That’s it. No new animal studies. No multi-year clinical trials. Just science that proves the generic behaves the same way in the body.
What’s the difference between an ANDA and an NDA?
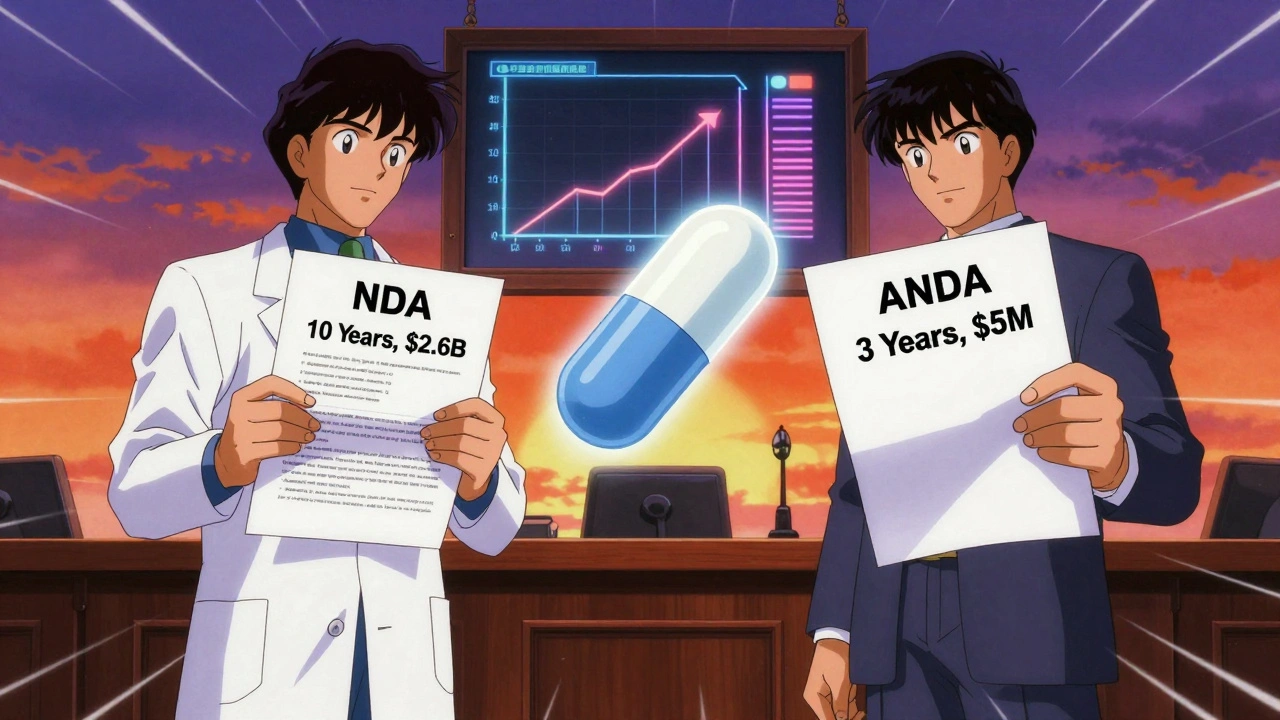
Think of the New Drug Application (the full application required for brand-name drugs to prove safety and efficacy from scratch) as building a house from the ground up. You need blueprints, permits, inspections, materials-all of it. That’s what the innovator company does when they develop a new drug. It takes 10-15 years and costs around $2.6 billion.
An ANDA is like buying a pre-built house that’s identical to the original. You don’t need to pour new foundations or install new wiring. You just need to prove the plumbing and electrical systems work the same. That’s why an ANDA takes 3-4 years and costs $1-5 million.
The FDA reviews both under the same safety standards. But only the NDA requires original clinical data. The ANDA leans on the brand’s data-legally and ethically-because the FDA already confirmed it’s safe and effective.
What gets approved through an ANDA?
Over 11,000 generic drugs have been approved through ANDAs as of 2023. That’s 90% of all prescriptions filled in the U.S. These include common meds like:
- Atorvastatin (generic for Lipitor)
- Metformin (for diabetes)
- Levothyroxine (for thyroid)
- Amlodipine (for high blood pressure)
- Apixaban (generic for Eliquis)
Each approved ANDA gets a unique six-digit number-like ANDA 214,455 for generic Eliquis. That number is public and searchable on the FDA’s Drugs@FDA database.
But not every drug can go generic this way. Complex products like inhalers, injectables, topical creams, and some transdermal patches often can’t be proven bioequivalent using standard methods. Those require special pathways or additional studies. The FDA has been working on new guidance since 2022 to handle these more complicated cases.
Why does the ANDA pathway matter?
Generic drugs save money. A lot of it.
When a brand-name drug’s patent expires, the first generic to file an ANDA often gets 180 days of exclusive market rights. That triggers price drops. Within a year, the generic version typically costs 80-85% less than the brand. In 2022 alone, 724 new generic approvals were projected to save $23.7 billion in healthcare spending.
Over the past decade, generic drugs saved the U.S. healthcare system over $2.2 trillion. In 2023, they accounted for 90.1% of prescriptions but only 23.4% of total drug spending. That’s a $313 billion annual savings for patients and insurers.
Without the ANDA pathway, most people couldn’t afford their medications. Insulin, blood pressure pills, antidepressants-all would be out of reach for millions.
Who files ANDAs and what’s the challenge?
The big players are Teva, Viatris (formerly Mylan), Sandoz, and Sun Pharma. Together, they control about 45% of the U.S. generic market. But hundreds of smaller companies also file ANDAs.
It’s not easy. The FDA rejects nearly 40% of first-time ANDA submissions. Common reasons:
- Manufacturing issues (32% of rejections)-poor quality control, inconsistent production, or unapproved suppliers
- Bioequivalence problems (27%)-inadequate study design, too few participants, or data that doesn’t meet the 80-125% range
- Labeling mismatches-minor differences in wording or formatting that don’t match the brand’s approved label
Companies with dedicated regulatory teams have a 42% first-cycle approval rate. Those without? Just 28%. The process demands precision. One typo in a batch record can delay approval for months.
Since 2017, all ANDAs must be submitted electronically through the FDA’s eCTD system. That cut administrative errors by 60%. Still, the learning curve is steep. New companies often spend 18-24 months training before submitting their first application.
What’s next for ANDAs?
The FDA’s new GDUFA IV agreement (2023) aims to boost first-cycle approval rates from 65% to 90% by 2027. That means faster access to generics.
More complex drugs are entering the pipeline. Nasal sprays, eye drops, and topical antifungals are now being targeted for generic approval. The FDA has released over 1,500 specific guidance documents to help companies navigate these tricky products.
But there’s a risk. Over 80% of generic drug ingredients are made in India and China. If supply chains break down-due to natural disasters, political issues, or quality failures-drug shortages follow. Experts warn this over-reliance creates systemic vulnerability.
Still, the path forward is clear. The Congressional Budget Office predicts generic drugs will save the U.S. $1.7 trillion between 2024 and 2033. The ANDA system isn’t perfect, but it’s working. It’s the reason your $150 brand-name pill became a $5 generic. And it’s why millions can still afford to stay healthy.
Frequently Asked Questions
Is an ANDA the same as a generic drug?
No. An ANDA is the application submitted to the FDA to get approval for a generic drug. The generic drug is the actual medicine you take. The ANDA is the paperwork that proves it’s safe and effective enough to be sold.
Do generic drugs work as well as brand-name drugs?
Yes. The FDA requires that generic drugs perform identically to their brand-name counterparts. Studies show over 97% of generic drugs are therapeutically equivalent. Millions of patients use generics safely every day. If a generic didn’t work the same, it wouldn’t be approved.
Can a brand-name company block a generic from entering the market?
They can delay it, but not stop it permanently. Under the Hatch-Waxman Act, a generic applicant must certify whether they’re challenging any patents on the brand drug. If they challenge a patent, the brand company can sue, triggering a 30-month stay on FDA approval. But if the generic wins the lawsuit or the patent expires, approval moves forward. Many generics enter the market after patent expiry, regardless of lawsuits.
Why are some generics more expensive than others?
Price depends on competition. If only one or two companies make a generic, prices stay higher. Once five or more manufacturers enter the market, prices drop sharply. Also, some generics are harder to make-like complex inhalers or injectables-and cost more to produce. That’s reflected in the price.
How long does it take to get an ANDA approved?
Under current FDA timelines, a standard ANDA review takes about 10 months. But if the application is incomplete or has issues, the FDA issues a "complete response letter" asking for fixes. That can add 6-12 months or more. Companies with strong regulatory teams and clean submissions often get approved faster.
Next steps if you’re researching generic drugs
If you’re a patient wondering why your prescription switched to a generic, you can check its approval status on the FDA’s Drugs@FDA database. Search by the brand name or generic name-you’ll see the ANDA number and approval date.
If you’re a student or professional in pharma, start by reading the FDA’s guidance on bioequivalence studies and the Orange Book. These are the foundation of the ANDA system. And if you’re considering entering the generic market, expect a long road-but one that’s worth it. Every approved ANDA means another person can afford their medicine.

Comments (8)
Jack Dao December 2 2025
Wow. Another one of those ‘generic drugs are just as good’ fairy tales. Let me guess-you also think tap water is the same as bottled mineral water? The FDA’s 80-125% bioequivalence range is a joke. I’ve seen patients crash after switching. The excipients? Totally different. The pill doesn’t dissolve the same. You think your body can’t tell the difference? Please.
Steve World Shopping December 2 2025
From a pharmacoeconomic standpoint, the ANDA pathway represents a paradigmatic shift in regulatory arbitrage, leveraging pre-existing NDA datasets to circumvent redundant clinical endpoints. The Hatch-Waxman Act essentially institutionalized regulatory capture by enabling oligopolistic generic manufacturers to exploit patent cliffs while minimizing R&D liability. Bioequivalence metrics are statistically porous-Cmax and AUC thresholds are not pharmacodynamically equivalent, merely pharmacokinetically proximate.
Rebecca M. December 3 2025
Oh sweet mercy, another 3,000-word essay on how generics are ‘just as good.’ 😌 Let me grab my tissues while I sob over the 85% price drop… while my insurance still charges me $12 for a 30-day supply of ‘generic’ metformin that gives me diarrhea like it’s a competitive sport. 🙃
Lynn Steiner December 3 2025
My dad died because he couldn’t afford his blood pressure med. Then they switched him to the generic and he had a stroke three weeks later. They told me it was ‘bioequivalent.’ Bullshit. I’ve seen the difference. And now they want to make us feel guilty for wanting the real thing? No. I won’t be quiet about this. 💔
Alicia Marks December 4 2025
Generics save lives. Period. 💪 If you can afford your meds, be grateful. If you can’t, thank the ANDA system. It’s not perfect-but it’s the reason millions aren’t choosing between food and insulin.
Paul Keller December 6 2025
While the structural efficiency of the ANDA framework is undeniably laudable from a public health economics perspective, one must acknowledge the systemic vulnerabilities inherent in the globalized API supply chain. The concentration of active pharmaceutical ingredient manufacturing in two geopolitical regions-namely India and China-introduces non-trivial systemic risk, particularly in the context of geopolitical instability, climate-induced logistical disruption, and quality control inconsistencies. The FDA’s recent GDUFA IV initiatives, while commendable, remain insufficient without parallel investment in domestic manufacturing capacity and diversified sourcing protocols. The cost savings, though monumental, are predicated on a fragile foundation.
Roger Leiton December 6 2025
Just checked my Eliquis generic-ANDA 214455. 🤯 That’s wild. I had no idea there was a public database for this. Also, the 180-day exclusivity thing? Genius. It’s like a race to the bottom… but in a good way. More companies = cheaper pills. 😎
Laura Baur December 7 2025
It is not merely a matter of therapeutic equivalence-it is a philosophical question of identity in pharmacology. If two substances produce statistically indistinguishable plasma concentration curves, are they truly identical? Or are we reducing the human body to a series of measurable variables, ignoring the subtle, unquantifiable nuances of individual physiology, microbiome variation, and psychosomatic response? The FDA’s binary approval model-pass/fail, bioequivalent/not-assumes homogeneity where none exists. We are not lab rats. We are not data points. And yet, we are expected to swallow these pills, and trust the numbers. This is not medicine. This is algorithmic healthcare. And it is profoundly dehumanizing.