Tag: generic drugs
Generic drugs save patients thousands over a lifetime by cutting chronic condition medication costs by 80-85%. Learn how generics work, why they're just as safe, and how to start saving now.
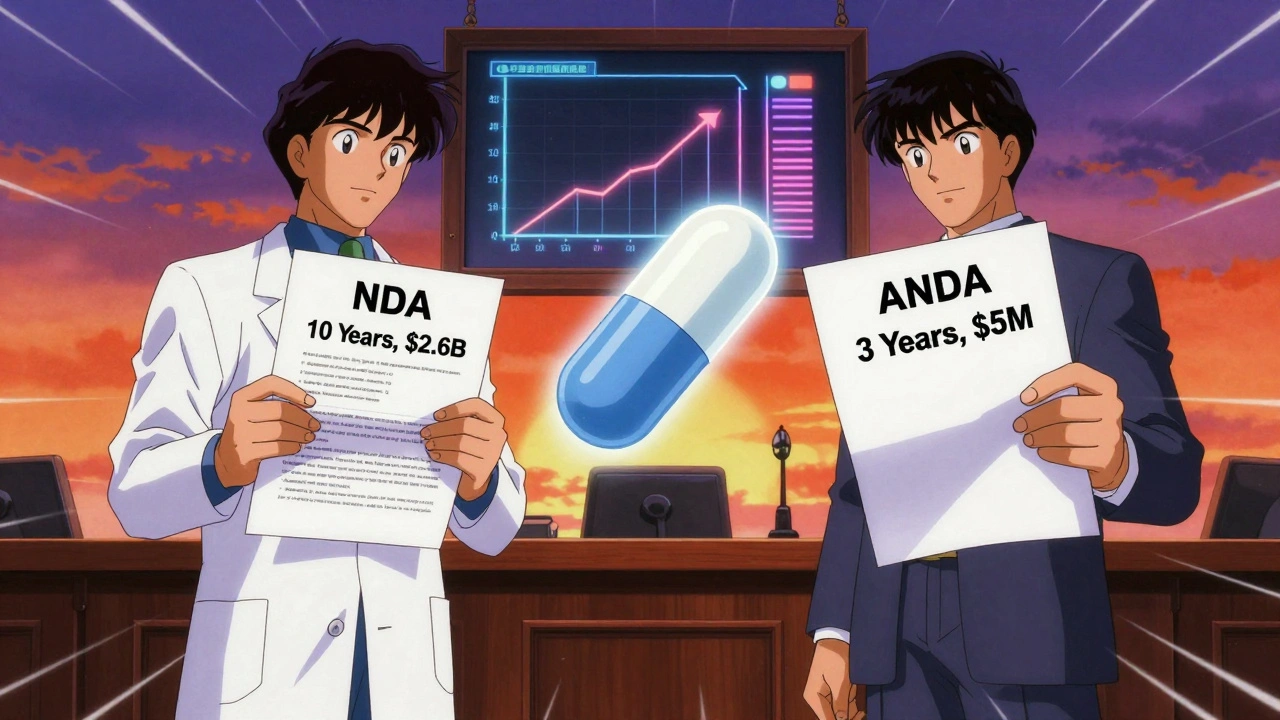
Paragraph IV certifications let generic drug companies challenge brand-name drug patents before launch, speeding up affordable medicine access. This legal tool under the Hatch-Waxman Act has saved consumers trillions since 1984.
Patent expiry means cheaper generics-but only if you plan ahead. Learn what patients and healthcare systems must do now to avoid cost traps, side effects, and supply shortages as hundreds of drugs lose exclusivity.
An ANDA is the FDA application that allows generic drugs to enter the market without repeating costly clinical trials. It's how 90% of U.S. prescriptions are filled at a fraction of the cost.
Narrow therapeutic index drugs require extreme precision in dosing. Learn why generic versions can be risky, how the FDA regulates them, and what steps you can take to stay safe when switching medications.
Despite generics making up 90% of prescriptions, many doctors still doubt their effectiveness. This article explores why providers hesitate, what data they need, and how real-world evidence is slowly changing attitudes.